What are HMOs (human milk oligosaccharides)?
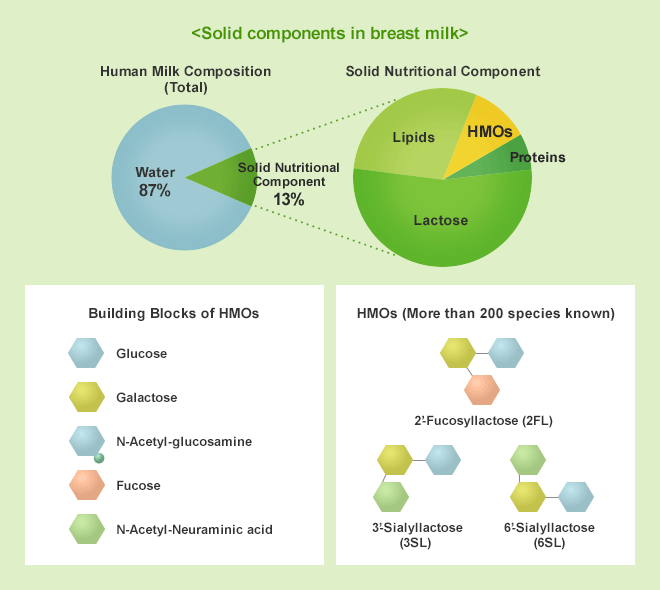
HMOs (Human Milk Oligosaccharides) are the general term for complex sugar molecules (oligosaccharide) occurring in human breast milk and represent the third most abundant solid components after lactose and lipids. To date, more than 200 HMOs have been identified in human breast milk.
* Oligosaccharides: saccharides with several monosaccharides attached. HMOs are mainly composed by glucose, galactose, fucose, N-acetylglucosamine, and N-acetylneuraminic acid.
HMOs are known to be important nutrient for infants because they are particularly abundant in human colostrum while bovine milk contains little or trace amounts of HMOs.
The function of HMOs
HMOs are prebiotics * that are nutrients for beneficial microorganisms, such as bifidobacteria. HMOs are not metabolized by human digestive enzymes and reach the large intestine intact. HMOs are metabolized by intestinal bacteria and exerts various physiological functions.
* Prebiotics: Substances that provide a selective nutrient source for microorganisms that are beneficial to the human body, and stimulate the growth and proliferation of microorganisms.
HMOs have the potential to support our health as functional ingredients, not just as a nutrient for infants.

Progress of HMO Development in Kyowa Hakko Bio
Mass production of HMOs was difficult due to their complex chemical structure. Therefore, it has not been possible to formulate them in infant formula for a long time. However, by applying our amino acid fermentation technology, we established a HMO production process and achieved mass production. 1
[Manufacturing process]
Saccharides (starting material) → Microbial fermentation → Sterilization → Purification → HMOs
| 1998 | Establishment of mass-production technologies for sugar chains by fermentation2 |
|---|---|
| 1999 | Received Nikkei BP Technology Award in Medical and Biotechnology section for "World's First Industrial-Level Production System Technology for Sugar Chains" |
| 2013 | Establishment of HMO plant scale manufacturing method |
| Present | HMO production facilities are in preparation. |
* Production under global food quality standards (e.g., HACCP, Food GMP, FSMA, FSC22000)
* To be compliant with Halal and Kosher requirements
(Planning to provide 2FL, 3SL and 6SL in 2022 and to expand our product line in the future)
References
- T. Endo, S. Koizumi, K. Tabata, A. Ozaki, Large-scale production of CMP-NeuAc and sialylated oligosaccharides through bacterial coupling. Applied Microbiology and Biotechnology, 53, 257-261 (2000)
- S. Koizumi, T. Endo, K. Tabata, A. Ozaki, Large-scale production of UDP-galactose and globotriose by coupling metabolically engineered bacteria. Nature Biotechnology, 16, 847-850 (1998)















